Introduction
Hypersensitivity reactions to chemotherapy—ranging from mild rashes to life-threatening anaphylaxis—can complicate treatment and require expert diagnosis and management.
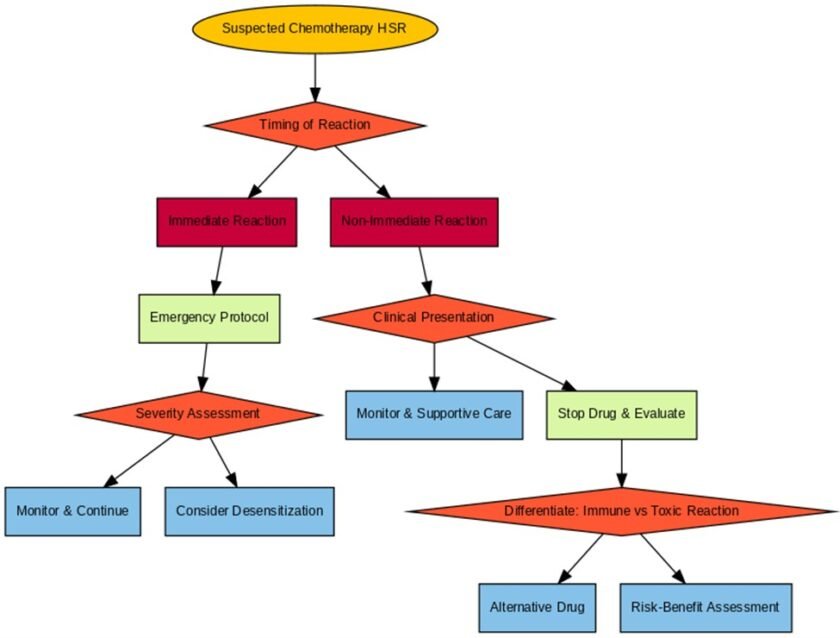
Immediate Reactions: Diagnosis and Management
Immediate hypersensitivity reactions to chemotherapies are relatively common and easier to confirm compared to non-immediate reactions. Symptoms such as urticaria, wheezing, or anaphylaxis often appear during or shortly after drug administration. In some cases, nausea, vomiting, or back pain may also occur as part of anaphylaxis symptoms, though these are less typical and can overlap with side effects of chemotherapy itself.
Key Diagnostic Tools:
• Skin Testing with Immediate Reading: Particularly useful for platinum-based drugs, providing evidence of IgE-mediated reactions.
• Basophil Activation Test: A recently introduced in vitro diagnostic tool that shows promise in helping to confirm immediate hypersensitivity reactions, though its use is not yet widespread.
Management Strategies:
• Immediate cessation of the drug and administration of emergency medications (e.g., epinephrine).
• Desensitization Protocols: Allow patients to continue chemotherapy safely. Recent studies confirm that the one-bag desensitization protocol is as effective as the traditional three-bag method while significantly reducing time requirements.
Non-Immediate Reactions: Diagnostic Challenges
Non-immediate reactions are harder to confirm due to their delayed onset and lack of reliable diagnostic tools. These reactions can include maculopapular rashes, severe cutaneous adverse reactions (SCARs), or drug-induced hypersensitivity syndromes.
Diagnostic Tools:
• Skin Testing with Delayed Readings or Patch Tests: Rarely used in chemotherapy-induced reactions due to limited sensitivity.
• In Vitro Tests: Tools like the lymphocyte transformation test (LTT) or interferon-gamma ELISpot assay are rarely positive because chemotherapeutic drugs are cytotoxic, often causing false negatives.
• Drug Provocation Testing: Less commonly performed due to the risk of prolonged and severe reactions, as well as the inherent toxicity of chemotherapeutic agents.
• Drug Desensitizations: In some mild cases of non-immediate reactions, desensitization protocols have been successfully performed, allowing patients to continue essential chemotherapy under close monitoring. This approach requires careful risk assessment and specialist involvement.
Key Considerations:
• Non-immediate reactions can sometimes mimic immune-mediated hypersensitivity but may be due to direct drug toxicity instead.
• Accurate diagnosis relies heavily on clinical history, patient symptoms, and disease progression.
Toxic Erythroderma in Chemotherapy
Non-immune-mediated reactions like toxic erythroderma are a well-recognized side effect of chemotherapies. These reactions can mimic immune-mediated hypersensitivity and present with phenotypes resembling Symmetrical Drug-Related Intertriginous and Flexural Exanthema (SDRIFE) or Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis (SJS/TEN)-like syndromes.
Chemotherapies Commonly Causing Toxic Erythroderma:
• Cytarabine (Ara-C)
• Capecitabine
• 5-Fluorouracil (5-FU)
• Doxorubicin
• Methotrexate
• Docetaxel and Paclitaxel
• Bleomycin
• Gemcitabine
• Etoposide
• Platinum-based agents (Cisplatin, Carboplatin, Oxaliplatin)
These reactions are not immune-mediated but result from the cytotoxic effects of chemotherapy on normal skin cells. Differentiating between immune-mediated hypersensitivity reactions and toxic drug effects is critical in the management of non-immediate reactions.
Differentiating Immune-Mediated Reactions from Toxic Effects
When a severe cutaneous reaction occurs, it is essential to differentiate between immune-mediated hypersensitivity and direct toxic effects of chemotherapeutic drugs. This step guides appropriate management:
• Immune-Mediated Reactions:
o Examples: Stevens-Johnson Syndrome (SJS), Toxic Epidermal Necrolysis (TEN), or Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS).
o Management: Immediate drug discontinuation, substitution with an alternative agent, and supportive care.
• Direct Toxic Effects:
o Examples: Toxic erythroderma, SDRIFE-like reactions.
o Management: Risk-benefit assessment of continuing the drug with supportive measures, including dose adjustment or symptom management.
Clinical evaluation, including timing, presentation, and patient history, is key to distinguishing these reactions. In vitro tests and skin testing may be less reliable due to the cytotoxicity of the drugs.
🧑⚕️ The Role of Shared Decision-Making in Chemotherapy Allergy Management
Shared Decision-Making and Patient-Centered Care
The complexity of hypersensitivity reactions to chemotherapy underscores the importance of shared decision-making. This involves:
- Thorough Communication: Discussing the risks and benefits of continuing chemotherapy after a reaction.
- Collaborative Planning: Working with the patient to determine the best course of action, whether it’s desensitization, switching drugs, or supportive care.
- Informed Consent: Especially in cases involving drug rechallenges or desensitization, patients should fully understand the potential risks and outcomes.
Key Takeaways
• Immediate reactions are easier to confirm with tools like skin testing and basophil activation tests. Desensitization (e.g., one-bag protocol) is highly effective in managing severe reactions.
• Non-immediate reactions pose significant diagnostic challenges due to limited test sensitivity and the toxic nature of chemotherapeutic drugs.
• Toxic erythroderma caused by chemotherapies can mimic immune-mediated hypersensitivity and requires careful clinical evaluation to avoid misdiagnosis.
Accurate diagnosis requires a comprehensive evaluation of the clinical course, patient history, and presenting symptoms. Shared decision-making between the healthcare provider and patient is essential for determining the best course of action.
References
- Pagani M, Bavbek S, Álvarez-Cuesta E, et al. Hypersensitivity reactions to chemotherapy: an EAACI position paper. Allergy. 2022;77(2):388-403.
- De Campos L, Giavina-Bianchi P, Acharya S, et al. Basophil activation test as a biomarker for taxanes anaphylaxis. Front Allergy. 2022;3:787749.
- Iglesias-Santamaría A, Castellano Copa P. Outcomes of a undiluted, one-bag desensitization protocol for chemotherapeutic agents. Ann Pharmacother. 2023;57(1):55-61.
- Chung SJ, Kang SY, Kang RY, et al. A new non-dilution rapid desensitization protocol successfully applied to all-grade platinum hypersensitivity. Cancer Chemother Pharmacol. 2018;82:777-785.
- Berges-Gimeno MP, Barra-Castro A, Fernandez-Lozano C, et al. Two Nonimmediate Reactions to Oxaliplatin and Docetaxel Confirmed by Lymphocyte Transformation Test and Treated With Successful Rapid Desensitization Procedures. J Investig Allergol Clin Immunol. 2025. doi: 10.1080/10802961.2025.2000894.
- Jimenez-Rodriguez TW, Cubo IL, De Jaime MN, et al. 686 Non-immediate hypersensitivity reactions in patients with gynecologic and breast neoplasms and management with desensitization. Int J Gynecol Cancer. 2024;34(Suppl_1):A265.2 [abstract].
- Lu A, Endicott A, Tan SY, et al. Toxic epidermal necrolysis-like toxic erythema of chemotherapy: 2 illustrative cases. JAAD Case Rep. 2021;15:56-59.
- Wolf R, Tüzün Y. Baboon syndrome and toxic erythema of chemotherapy: fold (intertriginous) dermatoses. Clin Dermatol. 2015;33(4):462-465.